What Are ADC Services and Why Are They Crucial for Biotech?
ADC services support the design, production, and testing of antibody drug conjugates for cancer and targeted therapies. They combine biology, chemistry, and manufacturing skills to turn complex science into usable medicines. For biotech teams, these services shorten development cycles, control quality risks, and enable progress from concept to clinic with greater confidence and regulatory alignment across global research and programs.
What ADC Services Include in Biotech Development
Antibody Discovery and Engineering Support
Antibody discovery support focuses on selecting targets, generating binders, and improving specificity. ADC service teams screen libraries, humanize antibodies, and engineer formats that balance affinity and stability. This work ensures the antibody delivers payloads precisely to diseased cells while reducing off target binding, immunogenicity concerns, and downstream development complications for biotech pipelines during early research planning and translational execution phases.
Linker and Payload Selection Strategies
Linker and payload selection shapes safety, potency, and differentiation of ADC candidates. Service providers evaluate cleavable and non cleavable linkers, cytotoxic mechanisms, and release profiles. By matching payload strength with tumor biology and antibody behavior, biotech companies can optimize efficacy while limiting systemic toxicity and improving therapeutic windows in clinical settings through data driven design and risk based decision frameworks.
Conjugation Process Development and Optimization
Conjugation process development defines how antibodies and payloads are reliably joined. ADC services design reaction conditions, control drug to antibody ratios, and improve reproducibility. Optimization reduces aggregation, preserves binding activity, and supports consistent batch quality. For biotech teams, robust conjugation methods simplify scale up and support smoother technology transfer to manufacturing partners under regulatory expectations and long term supply planning.
The Role of ADC Services Across the Drug Development Pipeline
Supporting Early-Stage Research and Feasibility
During early stage research, ADC services help validate targets and assess feasibility. Providers generate proof of concept material, conduct in vitro studies, and refine molecular designs. This support allows biotech teams to make informed go or no go decisions, conserve funding, and prioritize candidates with clearer paths toward differentiation and future development milestones across discovery programs and internal review processes.
Enabling Preclinical Development and IND Readiness
ADC services play a central role in preclinical development and IND preparation. Teams support toxicology studies, bioanalysis, and CMC documentation aligned with regulatory expectations. By generating reproducible data and scalable processes early, biotech companies reduce surprises, strengthen submissions, and approach regulatory interactions with greater confidence and technical clarity during agency meetings and formal review timelines for global development strategies alignment.
Scaling Up for Clinical and Commercial Needs
As programs advance, ADC services support scale up for clinical trials and commercialization. Providers adapt processes for larger batches, ensure supply consistency, and manage technology transfer. This capability helps biotech companies meet growing demand, control costs, and maintain product quality while transitioning from pilot production to validated manufacturing environments under GMP standards and long term capacity planning objectives globally aligned.
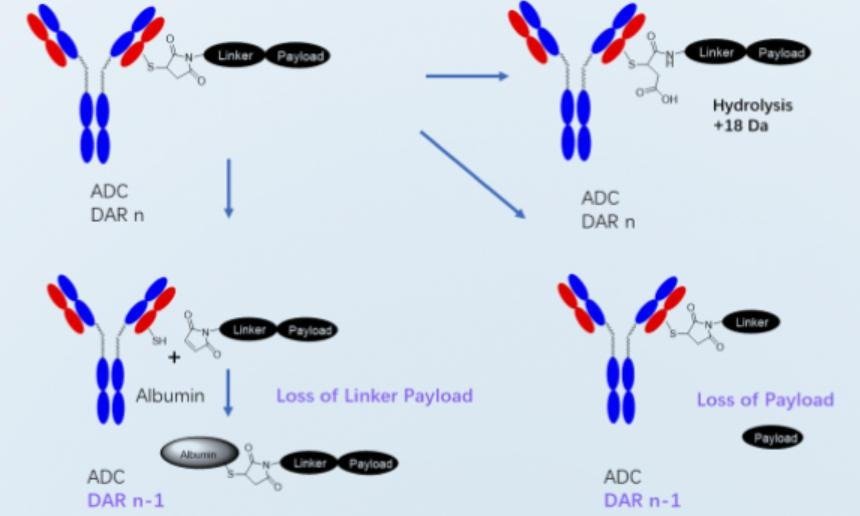
Key Technologies and Capabilities Behind ADC Services
Site-Specific Conjugation and Analytical Methods
Site specific conjugation technologies improve control over ADC structure and behavior. Services apply engineered residues, enzymatic methods, and advanced analytics to achieve uniform drug attachment. These approaches enhance stability, predictability, and safety profiles. For biotech developers, site specific methods support better comparability, clearer structure activity relationships, and more reliable clinical outcomes across diverse indications and development programs worldwide today.
See also: Skin Health vs. Skin Aesthetics: What Melbourne’s Best Clinics Are Getting Right
Stability, Characterization, and Quality Control
Stability and characterization services ensure ADC products remain safe and effective. Providers perform forced degradation, shelf life studies, and detailed physicochemical analysis. Quality control testing verifies identity, purity, and potency throughout development. For biotech companies, these capabilities reduce regulatory risk and support consistent performance from preclinical studies through late stage trials under evolving guidelines and global compliance expectations frameworks worldwide.
Integrated ADC Platforms and Workflow Efficiency
Integrated ADC platforms combine multiple services into coordinated workflows. They link discovery, conjugation, analytics, and manufacturing under unified project management. This integration improves communication, reduces delays, and limits handoff errors. For biotech teams, streamlined workflows increase efficiency, improve data continuity, and allow scientists to focus on decision making rather than vendor coordination across complex programs and fast moving development timelines.
Why ADC Services Are Critical for Biotech Companies
Reducing Development Risk and Time to Market
ADC services reduce development risk by applying proven methods and experience. Established processes prevent common failures in conjugation, stability, and scale up. By outsourcing complex tasks, biotech companies save time, avoid costly rework, and advance assets faster. Shorter timelines can improve competitiveness, investor confidence, and overall chances of reaching patients within crowded oncology markets and funding constrained environments globally today.
Accessing Specialized Expertise and Infrastructure
ADC development requires specialized skills, equipment, and facilities. Many biotech firms lack in house capabilities for complex conjugation and analysis. ADC services provide access to experienced scientists, validated technologies, and compliant infrastructure. This access allows smaller teams to pursue advanced programs without heavy capital investment or long internal build out timelines while maintaining focus on core scientific innovation goals strategically.
Improving Therapeutic Index and Clinical Success Rates
Optimizing the therapeutic index is central to ADC success. ADC services help balance potency and safety through better design and control. Precise conjugation, stable linkers, and thorough testing improve target delivery and limit toxicity. For biotech companies, these improvements increase clinical response rates and support more sustainable long term development strategies across diverse patient populations and indication types globally evolving.
Conclusion
ADC services have become essential partners for biotech innovation. They bring structure, expertise, and efficiency to a complex development model. By supporting every stage from discovery to commercialization, these services help companies manage risk and unlock value. For biotech growth, strategic use of adc services can define competitive advantage and long term success within evolving markets and demanding regulatory landscapes.